Limonene is a highly common scent ingredient and solvent naturally occurring in the rind of citrus fruit. This colorless liquid is often used for its light, fresh and sweet citrus odor in household and personal care products. In some cases, it is also used for its penetration-enhancing effects for skin care.
However, when limonene oxidizes not only can the odor turn rancid—it can go from beneficial to skin irritant. So careful quality control over this ingredient can mean the difference between sellable product and costly recalls.
Challenge
Manufacturing often depends on proper date of manufacture and expiration date labeling to ensure the freshness of ingredients. However, improper storage or container conditions can cause air exposure which then causes the undesirable oxidation.
Although fully oxidized limonene can be quite easy to identify, there is a degree of subjectivity between ‘good’ and ‘acceptable’ – as partially oxidized limonene can present the negative product effects without necessarily displaying the extremely pungent rancid odor.
Providing an objective reading of the olfactive quality of a limonene ingredient can provide manufacturing a traceable, reliable way to document the ingredient quality in production. This reduces the risk around safety or product quality recalls for this particular defect.
Solution
Digital olfaction from Aryballe enables manufacturers to validate ingredient quality when accepting raw materials and before adding them to the production line. Easy-to-implement quality standards can be defined by quality teams and measured by production teams. With digital olfaction tools, manufacturing managers can quickly and easily determine out-of-spec ingredients and flag potential quality control issues before they enter the manufacturing process.
The tests performed with the NeOse Advance were used to classify two samples—limonene that met quality specifications and oxidized limonene (ie out of spec).
Each sample was prepared in 60 ml vials in duplicate at ambient temperature for 5 minutes. After this, each sample was tested by the NeOse Advance using the HeptaValve Mini and the Aryballe Suite for data acquisition (Figure 1). Five replicates were taken for each sample, waiting two minutes between measurements. Before measuring samples, a minimum of five minutes of waiting time was allowed to ensure complete formation of the headspace in the vial.

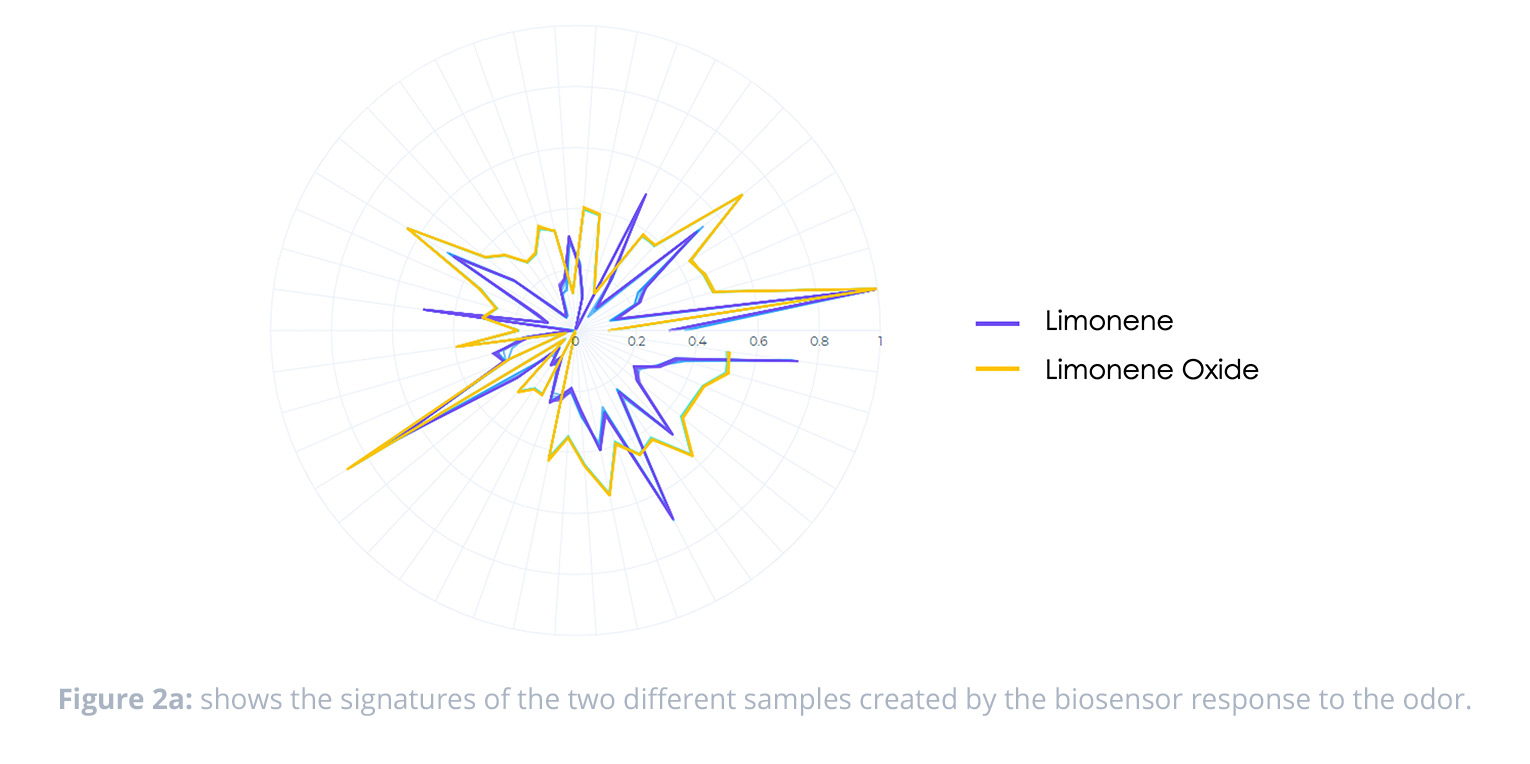
The signatures captured by the NeOse Advance showed distinct differences in peptide response across the two classifications of samples. We can see further evidence of the discrimination of the samples in the PCA—which shows very good clustering and a high degree of separation between the samples.
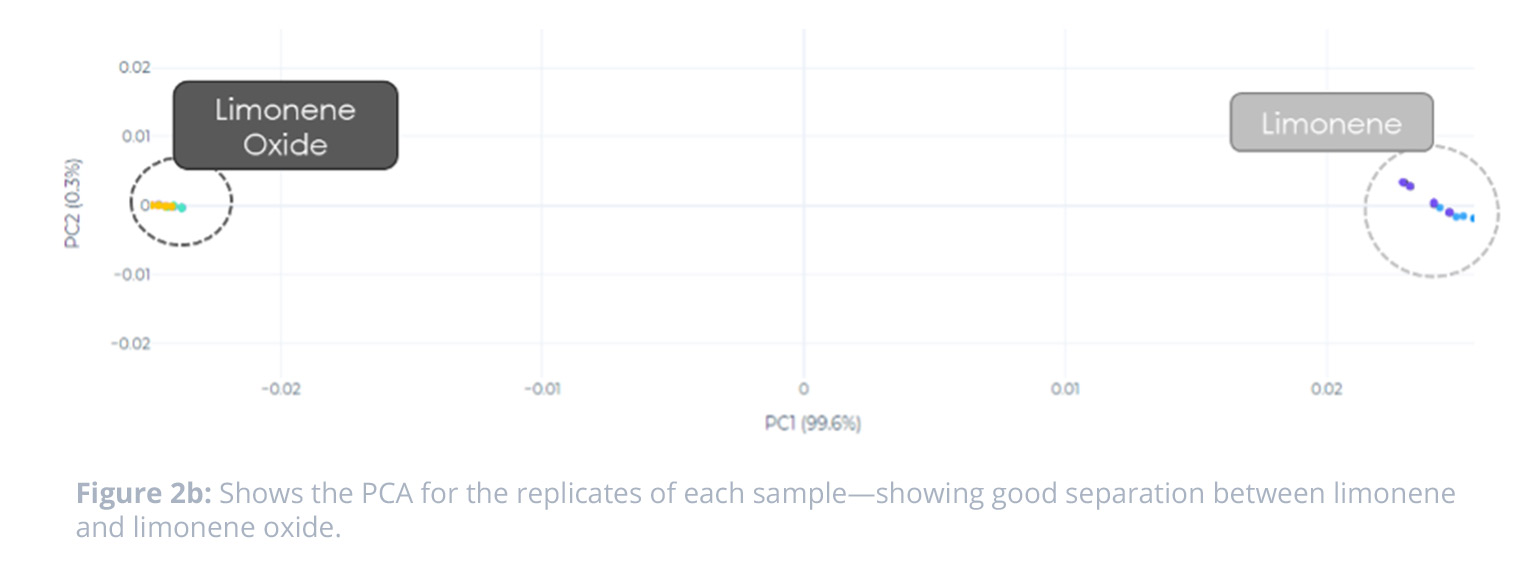
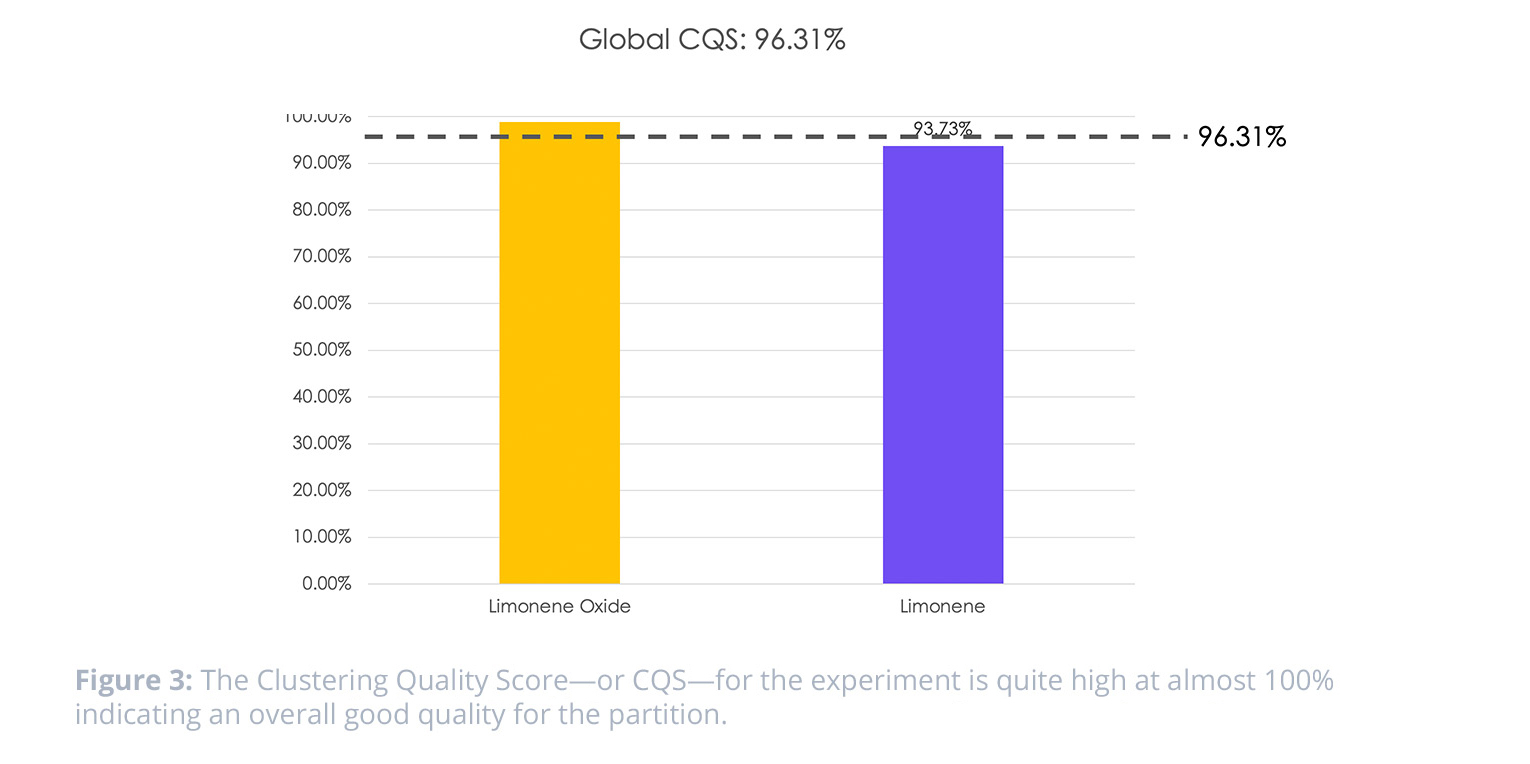
When looking at the Clustering Quality Score (CQS) for the data, we see that there is good quality of the partition for the samples. This indicates that there is tight cluster for the replicates of one sample, and separation between the two sample types—limonene and limonene oxide.
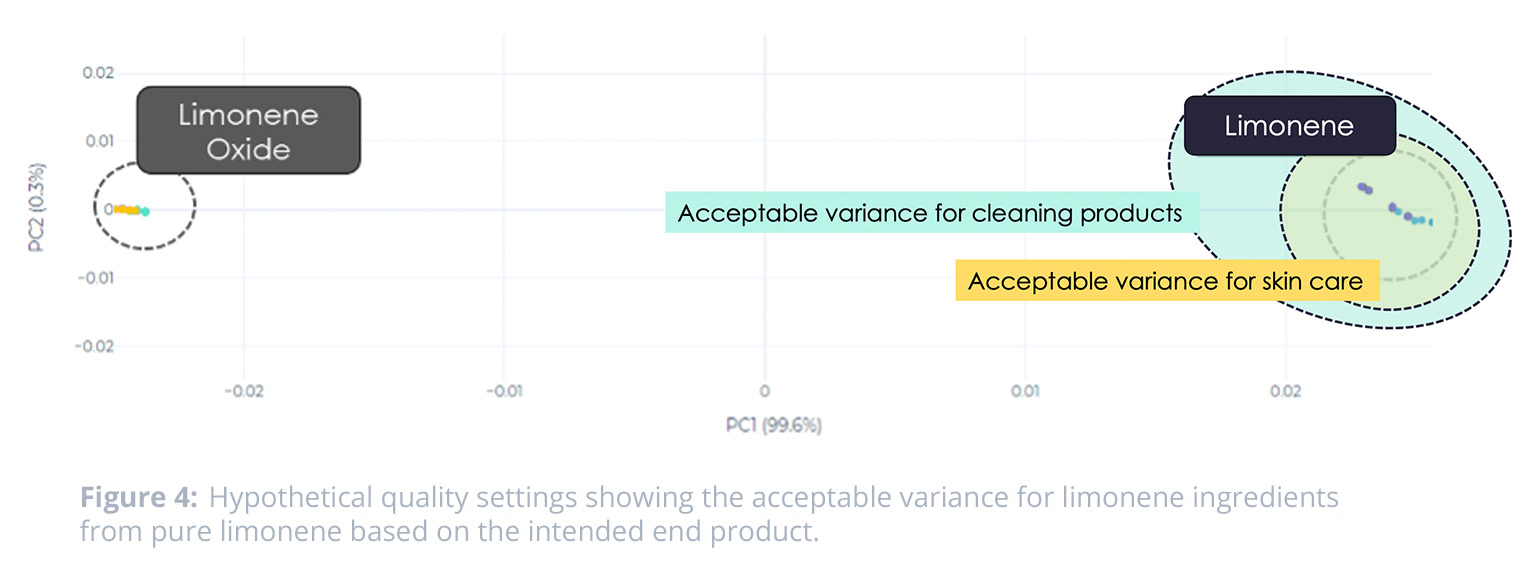
In practice, quality teams can use this data to define “good” and “bad” levels of this ingredient—and then define the acceptable threshold for an incoming sample between these two points. This allows the quality team to give the manufacturing team numerical standards—and also allows for a level of variance in what is acceptable based on the product line and the function of the limonene ingredient for that product.
Conclusion
The results of the tests showed that the NeOse Advance can be used to distinguish differences in the limonene samples that can create insights for manufacturing decisions based on objective, reliable odor data. By integrating digital olfaction into flavor, fragrance and food industry workflows, organizations can use traceable, consistent odor data for decision making.

